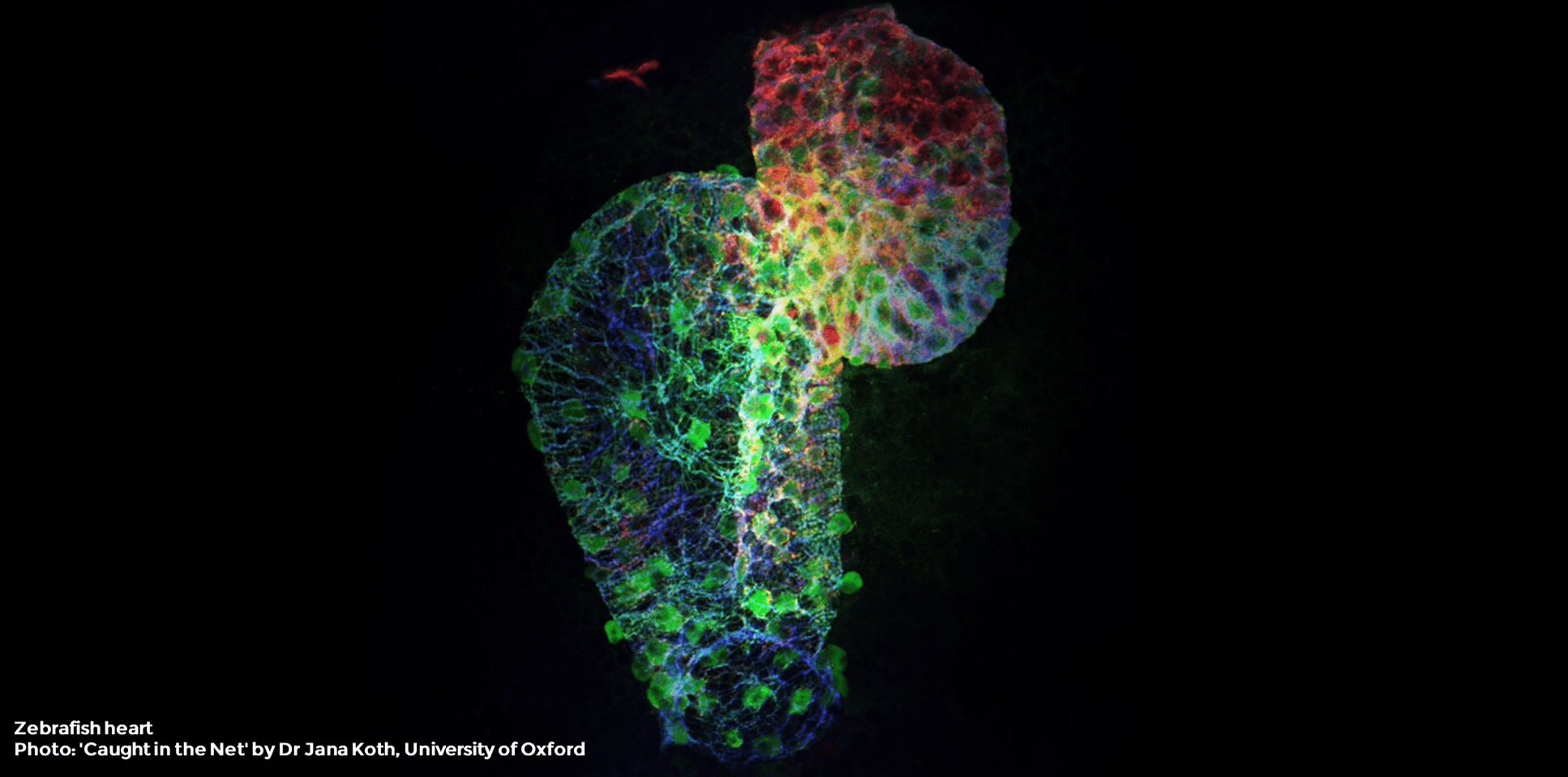In this series of articles, we review some of the key scientific publications of 2020 which included zebrafish in their studies. This review will cover an article published in Nature Communications on January 30th, 2020: Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair1.
This article challenges the current model of scarring after a heart attack and implicates macrophages, a type of white blood cell of the immune system, as direct contributors to collagen deposition during this process.
Heart attack and heart failure
Over a million of Americans suffer a heart attack each year2. Heart attacks, more precisely named as myocardial infarctions, occur when the flow of oxygen-rich blood to a section of heart muscle decreases or stops, causing damage to the heart tissue3. If this blockage is not treated quickly, the portion of the heart affected may die, and a process of scar formation takes place.
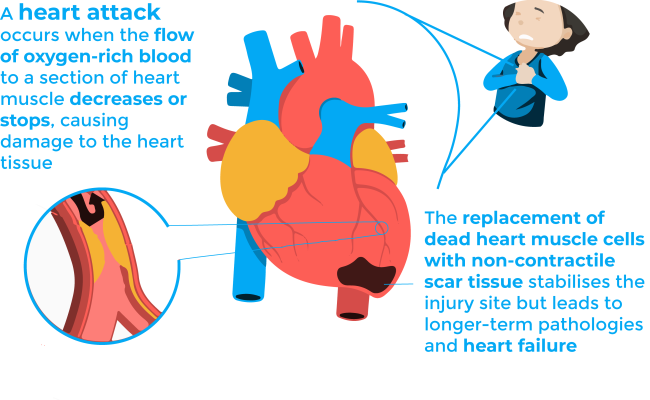
The replacement of dead heart muscle cells with non-contractile scar tissue stabilises the injury site but leads to longer-term pathologies and heart failure3. The latter refers to the situation when the heart is unable to pump sufficiently to maintain blood flow to meet the body tissues’ need for metabolism 4,5. It is a potentially fatal condition, and it is the leading cause of both hospitalization and readmission amongst older adults. Thus, understanding the biological mechanisms underlying scar formation is of great importance, since the fate of patients who survive the initial infarction could be determined by some properties of the healing scar, such as its size, location, composition, structure and mechanical properties.
Scarring process after a heart attack
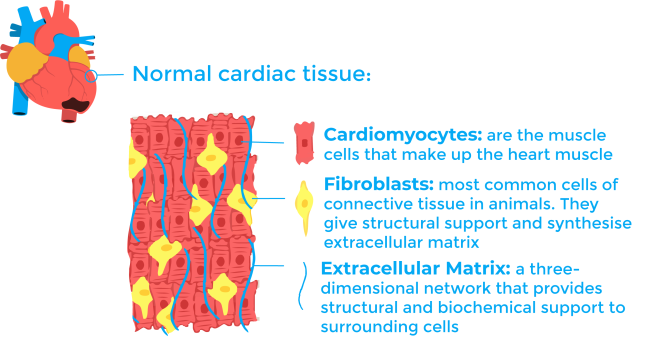
To understand the process of scar formation, it is important to first describe the cardiac tissue. The heart is a muscular compartment composed of large cardiomyocytes (heart muscle cells) and an interstitial compartment. The interstitial compartment contains extracellular matrix and vasculature, each having their own distinctive cellular and molecular composition. Once the cardiomyocytes die during a heart attack, the process of scarring begins. The current model for scarring is generally divided into three stages: inflammation, fibrosis, and remodelling6,7.
The first inflammatory phase begins as the dying cardiomyocytes start a molecular signalling cascade wherein a variety of inflammatory cells including neutrophils, macrophages and lymphocytes invade the ischemic zone6,7. These cells play a central role in the wound healing process both by remodelling the tissue and continuing with the signalling cascade. They, together with dying cardiomyocytes, secrete and activate some enzymes called MMP (matrix metalloproteinases) that will degrade cell and matrix material in the ischemic zone6,7. This step is necessary for example so that cells as macrophages can reabsorb the dying tissue.
During the second phase, called the fibrotic phase, another cell type enters the process: myofibroblasts. These cells are not residents of normal cardiac tissue and appear at the infarct site playing a major role during the fibrotic phase. They are responsible for collagen synthesis6,7. Collagen is the main structural protein in the extracellular matrix and its deposition is essential for scar formation.
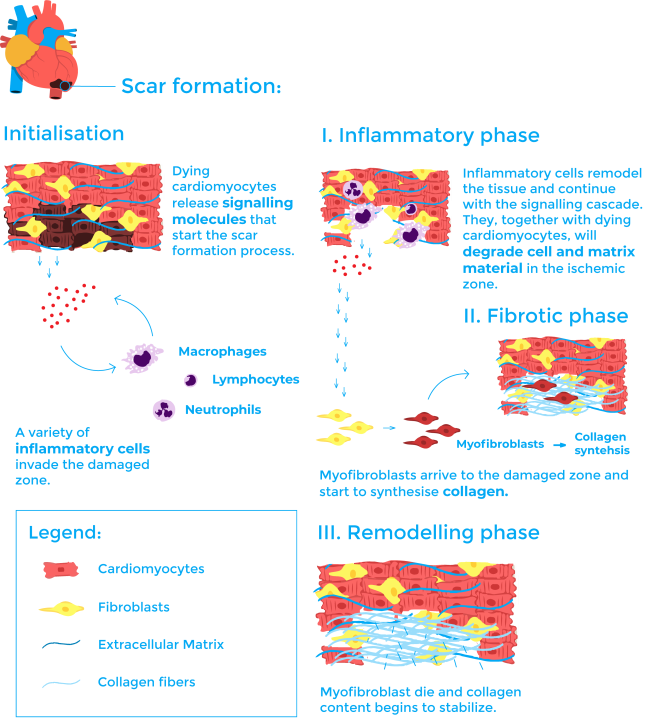
The final stage is known as the remodelling phase. In this phase, myofibroblast go through apoptosis (a mechanism of programmed cell death) and collagen content begins to stabilise. The scar matures thanks to a steady increase in collagen crosslinking 6,7.
Macrophages and their different stages
The reviewed article studies the role of macrophages during scar formation. Macrophages are a type of white blood cell of the immune system highly specialised in removing dying or dead cells and cellular debris8. Macrophages engulf and digest anything that does not have on its surface proteins that are specific to healthy body cells. Macrophages are the predominant immune cell type in the cardiac tissue after a myocardial infarction. They infiltrate from the spleen and bone marrow9.
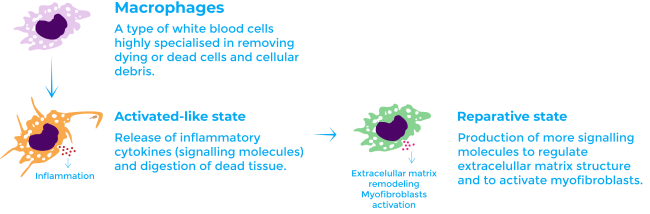
During the initial inflammatory phase, macrophages adopt a classically activated-like state. In this state, they release inflammatory cytokines (signalling molecules) and digest dead tissue. Afterwards, during the fibrotic phase, they transition to a reparative state in which they produce more signalling molecules to contribute to fibrosis: they regulate the balance of MMP and activate myofibroblasts1,10.
Until now it had not been reported that macrophages synthesise collagen during fibrosis and this function was thought to be exclusive of myofibroblasts. However, the results of the article strongly suggest a direct contribution in collagen synthesis by macrophages1.
Zebrafish as a model for heart repair
Two different models are used in the reviewed article: mice and zebrafish. Mice have long been a well-established model for myocardial infarction. In contrast, zebrafish have become increasingly important in this field during the last decades.
The zebrafish adult heart even if it is smaller and simpler than the mammalian heart (it has one atrium and one ventricle) has a histological and structural composition very similar to that of other vertebrates11. Besides, due to the ease of genetic approaches, and to the availability of different pathway reporter lines, zebrafish have been quickly accepted as a very useful system for studying molecular mechanisms of physiological processes.
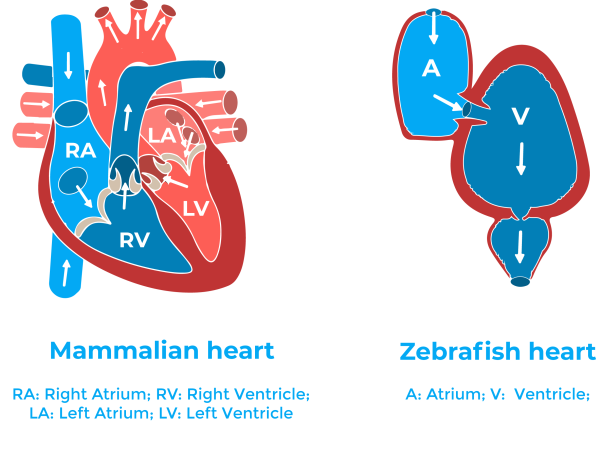
It is important to mention that differently than in humans, the adult zebrafish heart regenerates after an injury11. In the study, they use two different injury models: resection of the ventricle, and cryoinjury. The former results in complete heart regeneration, without stable scar formation; while in the latter, the heart regeneration is delayed, coincident with massive cell death and scar formation1. Therefore, being more closely to the human heart post-myocardial infarction. Interestingly, in neonatal mice the heart can regenerate too. The regeneration in these rodents occurs during a temporal window which extends to one week after birth12.
Collagen deposition by macrophages
Effective cardiac regeneration depends not only on the degree of regenerative competence of the injured heart but also on the modulation of the transition from a tissue repair phase to a regenerative phase. Macrophages are integral to both repair by scar formation and tissue regeneration. Since factors that drive them towards either a repairing or regenerative phenotype are largely unknown, the authors analyse the macrophage response after different cardiac injuries in mice and zebrafish.
Among other results, their experiments evidenced that macrophages are directly implicated to collagen synthesis during the scarring process1. The experiments they performed were of two types: the first consisted of transferring collagen-labelled macrophages to the wound site. These macrophages were modified so that the collagen synthesised by them was labelled and could be recognized. And a second type of experiments where they transferred macrophages unable to synthesise collagen. With these experiments they saw that there is labelled-collagen deposition within the forming scar after transferring collagen-labelled macrophages, and that the transfer of collagen-depleted macrophages results in a significant reduction in scar formation1.
Conclusion
The work described in Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair studies the context of cardiac repair and regeneration and provides significant insight into the cellular and molecular basis of scar formation. It challenges the current model for scarring by positioning macrophages as key players that contribute directly to scar formation by autonomous collagen deposition. Importantly these results were similar in mice and zebrafish, meaning that this mechanism is conserved across species.
Understanding the repair and regeneration mechanisms of the heart after an injury is essential for the development of new therapies for patients suffering from acute myocardial infarction. Even if there are still some questions to solve, such as the relevance of macrophage-derived collagen production during scarring processes, this study gives a new point of view and opens the door to new approaches for the development of new therapies.
References
- Simões F.C. et al.(2020) Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nature Commun https://doi.org/10.1038/s41467-019-14263-2
- Heart Diseases Facts (CDC). Retrieved May 25, 2021. https://www.cdc.gov/heartdisease/facts.htm
- What Are the Signs and Symptoms of Coronary Heart Disease? (NIH). Retrieved May 25, 2021. https://web.archive.org/web/20150224034615/http://www.nhlbi.nih.gov/health/health-topics/topics/cad/signs#
- Puggia I., et al. (2018) Chapter 1 – Molecular and Cellular Mechanisms in Heart Failure, Heart Failure in the Child and Young Adult, Academic Press. https://doi.org/10.1016/B978-0-12-802393-8.00001-6.
- Metra M., Teerlink J.R. (2017) Heart failure, The Lancet. https://doi.org/10.1016/S0140-6736(17)31071-1
- Richardson W.J., et al. (2015) Physiological Implications of Myocardial Scar Structure. In Comprehensive Physiology, R. Terjung (Ed.). https://doi.org/10.1002/cphy.c140067
- Sun Y., Weber K.T. (2000) Infarct scar: a dynamic tissue, Cardiovascular Research, https://doi.org/10.1016/S0008-6363(00)00032-8
- Macrophages (British Society for Immunology). Retrieved May 25, 2021 https://www.immunology.org/public-information/bitesized-immunology/cells/macrophages#:~:text=Macrophages%20are%20specialised%20cells%20involved,cytokines)%20that%20activate%20other%20cells.
- Ginhoux F., Guilliams M. (2016) Tissue-resident macrophage ontogeny and homeostasis. Immunity. https://doi.org/10.1016/j.immuni.2016.02.024
- Zhang, Yang M., Ericsson A.C. (2021) Function of Macrophages in Disease: Current Understanding on Molecular Mechanisms. Frontiers Immunology. https://doi.org/10.3389/fimmu.2021.620510
- Beffagna (2019) Zebrafish as a Smart Model to Understand Regeneration After Heart Injury: How Fish Could Help Humans. Frontiers Cardiovascular. https://doi.org/10.3389/fcvm.2019.00107
- Porrello E.R. et al. (2011) Transient regenerative potential of the neonatal mouse heart. Science. https://doi.org/10.1126/science.1200708