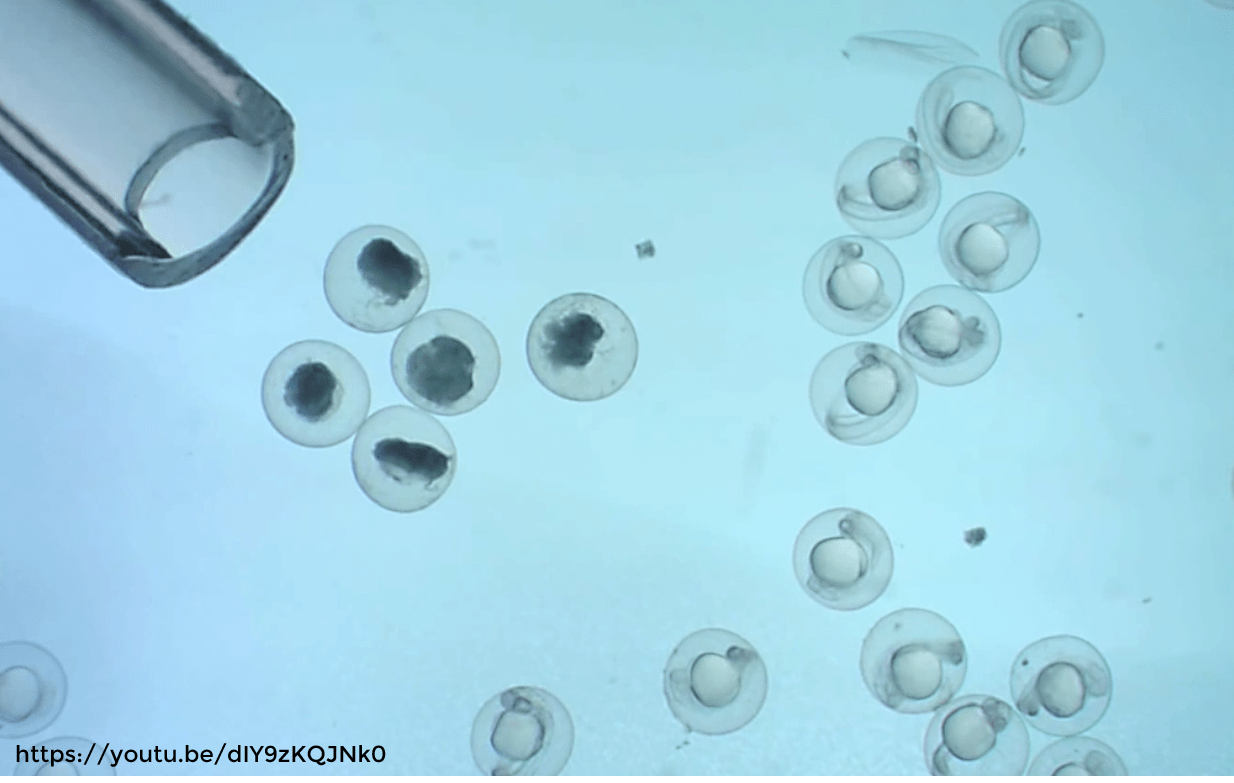
Over the past 20 years, the use of zebrafish in the lab has dramatically increased. In 2000, PubMed displayed 657 articles when searching for “zebrafish”. In 2020, that number shot up to 3’979 publications (1). This is most likely due to the many advantages zebrafish represent as an animal model (and which are resumed in our article What are zebrafish). Particularly, their high breeding capacity is a very interesting asset for experiments requiring large sample sizes. Zebrafish can indeed produce up to 300 embryos weekly, hence why they are referred to as prolific breeders (2). Although a large batch of embryos is preferable for statistical analysis, this also requires a lot of work for egg processing and sorting.
In this article we will address the importance of embryo processing and egg sorting, particularly the sorting of fertilised against non-fertilised eggs in order to retain only healthy embryos for the experimental task. To understand this sorting process, we will also briefly describe the steps of egg fertilisation and embryo development in zebrafish.
Breeding and mating behaviours
Zebrafish are widely recognised as prolific breeders due to their high breeding capacity. Although in their natural habitat they usually breed between April and August, in a laboratory setting associated with good reproduction conditions they can breed all year round and lay up to 300 eggs a week. It has been shown that in the wild, breeding occurs in shallow spaces and therefore such environments should be incorporated in laboratory set-ups. Additionally, circadian rhythms seem to play an important role in the initiation of breeding behaviours, which often correlate with the onset of dawn light (3).
It has been demonstrated that zebrafish are sexually stimulated by specific concentrations of pheromones which lead to several courtship behaviour mechanisms in male and female zebrafish (4).
Olfactory cues are triggered and initiate synchronised mating behaviours (5). Males initially chase and tail-nose female fish before encircling and swimming in a zig-zag motion next to them and quivering along their side. Females approach and escort the male fish, present themselves by halting before males and lead the swim through the tank before laying eggs.
Well-regulated courtship behaviours in males and females are essential for coordinated release of eggs and sperm leading to egg fertilisation. Following egg deposition, eggs must be rapidly collected to avoid consumption by parents. Thus, most laboratories use a mesh or slotted barrier to separate sinking eggs from parents (3). Specific breeding vessels have also been thought to collect large numbers of developmentally synchronised embryos in a short window of time (6). Once collected, eggs must rapidly be sorted.
Egg sorting: fertilised versus unfertilised eggs
A fertile female can release over a thousand oocytes during oviposition (3). It is obvious that not all of the eggs will be fertilised or lead to healthy developing embryos. There is indeed a high mortality peak in zebrafish embryos following fertilisation, known as the Embryonic Mortality Effect (EEM) (7). In 15% of cases, mortality arises during the 75 first hours post-fertilisation (7) and dead embryos become opaque, making them easy to identify.
The physical appearance of the egg is a good indicator of the egg’s health: if the egg is transparent and shiny it probably contains a healthy developing embryo whereas if the egg is opaque and white (or black, depending on the egg’s illumination. With the light from above the opaque cells appear white. On the contrary with light from below the sample, they appear black), the eggs is most likely unfertilised or contains a dead embryo (7). Therefore, sorting eggs according to their colour appears to be a good strategy. However, it is often assumed that all white eggs are unfertilised although this is not the case. As a matter of fact, a white egg can be one of three kinds (8). First of all, the egg might indeed simply not have been fertilised and as a result became white. Others might have been fertilised but did not develop. Finally, some eggs might have been fertilised and the embryos began to develop but died early on (8).
While removing dead embryos has a significant importance, as we will discuss further on, retrieving unfertilised eggs should be done quickly following a mating event. To understand how eggs can be distinguished based on morphological features, it is worth reminding a few important steps which occur during embryogenesis.
45 minutes post-fertilisation, the embryo undergoes meroblastic cleavage and each cell divides synchronously (3). A cell mass develops at the animal pole and gives rise to the blastula. After 10 cell division cycles, development reaches the mid-blastula transition point and cells begin to divide asynchronously (3). A few cycles later, the blastula spreads to completely surround the egg yolk. This is also known as epiboly (3). After 10 hours post-fertilisation, epiboly is completed and the fish head is oriented towards the animal pole and the tail bud towards the vegetal pole. Segmentation, neurulation and organogenesis occur successively until 36 hours post-fertilisation and the fish will hatch between 48- and 72-hours post-fertilisation (3).
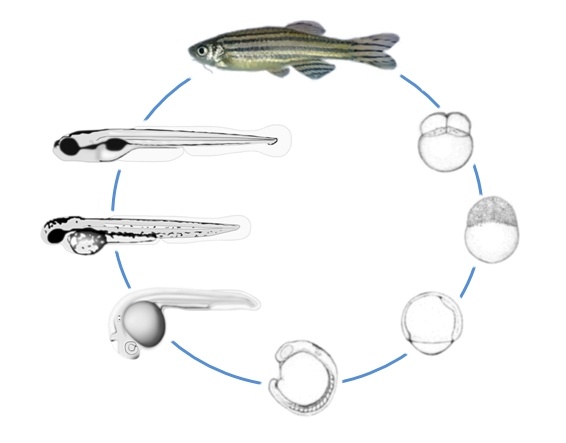
Figure 1 (10): stages of zebrafish embryo development. Cells divide and multiply at the animal pole. Cells divide synchronously until the mid-blastula transition point. During epiboly the cells surround the egg yolk until the head and tail are oriented to the animal and vegetal pole respectively (3).
Distinguishing fertilised from non-fertilised eggs is a difficult task. Initially, they cannot be distinguished as both eggs acquire an animal-vegetal polarisation (7). However, at 60 minutes post-fertilisation, two identical cells are visible at the egg yolk surface of fertilised eggs as blastomeres begin cell division. Blastomeres divide synchronously over the following hours until individual cells can no longer be distinguished as they get too small and no cell growth is happening. Unfertilised eggs remain at a single cell stage and no cell division occurs. Thus, based on morphological features, fertilised eggs can be distinguished from unfertilised eggs as fertilised eggs are characterised by the presence of two identical “humps” whereas unfertilised eggs only have one (7). The single hump on unfertilised eggs will swell and acquire an irregular shape. About 8 hours after having been released, the egg will turn white (7). Additionally, unfertilised eggs will lose cells to the perivitelline space during the 16 or 32 cell stages. This can also occur in fertilised eggs indicating mechanical stress which will lead to embryo death (7).
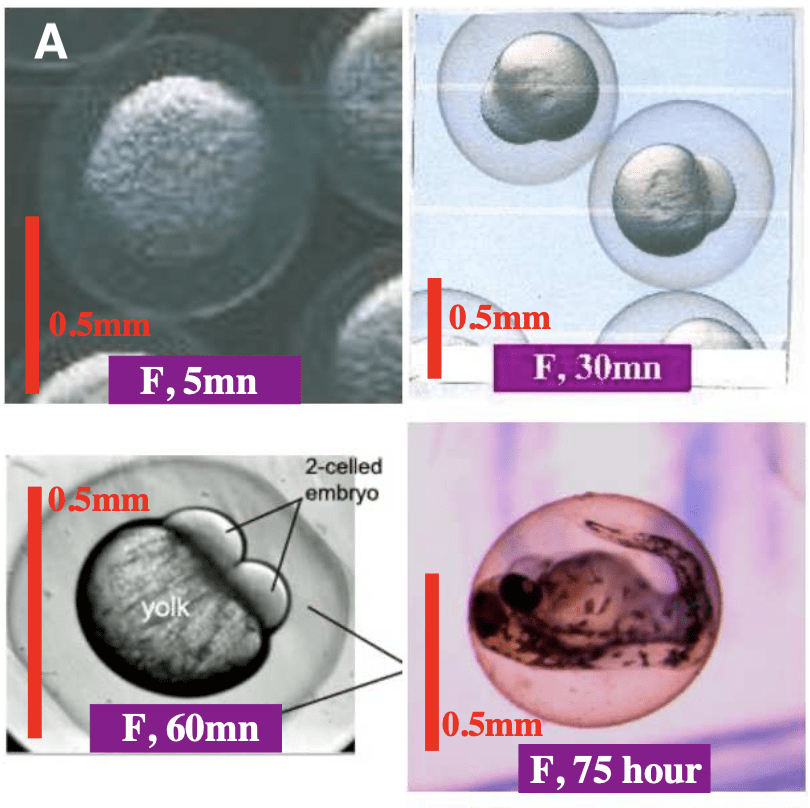
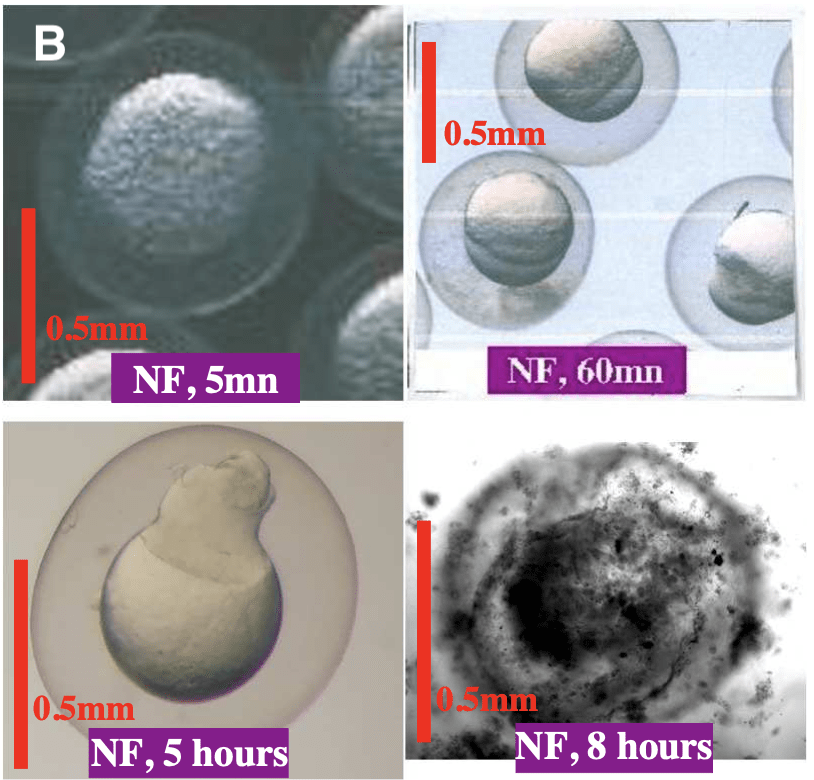
Figure 2: (A) Fertilised egg at 5 minute, 30 minute, 60 minute and 75 hours post fertilisation. 5 minutes post-fertilisation the fertilised egg looks identical to the unfertilised egg. After 30 minutes the initial embryonic cell is visible. After 60 minutes, cell division has began and two cell humps are present at the yolk surface. At 75 hours post- fertilisation the embryo is nearly ready to hatch. (B) Non-fertilised egg at 5 minute, 60 minute, 5 hours and 8 hours post fertilisation. 5 minutes post-fertilisation the unfertilised egg looks identical to the fertilised egg. At 60 minutes post-fertilisation a single hump is visible at the egg surface. 5 hours post-fertilisation the hump grows and acquires an irregular shape. The non-fertilised egg becomes black at 8 hours post-fertilisation. (7)
Egg sorting: why is it important?
Retrieving and sorting eggs after a spawning event and removing the unfertilised or dead ones is of high importance for maintaining optimal growth conditions and promoting the well-being of growing embryos. Optimal conditions for normal zebrafish embryo development depend on oxygen, temperature, debris, pH and several other factors (5). A lack of oxygen or an unsuitable temperature can lead to embryo death, usually during the morula stage.
Although embryos develop within a protective egg shell, they are not safe from all attacks. Indeed, some bacteria or fungus remain a threat to the embryos as they are capable of attacking and destroying the egg shell. Contaminated hatching water stimulate bacterial proliferation among the eggs which secrete enzymes to degrade the egg shells, leading to premature hatching and death. Similarly, fungi spores can develop and spread over dead eggs and eventually spread to healthy eggs, compromising embryo development. Therefore, removing unfertilised eggs or dead embryos is crucial to prevent batch contamination as spoiled eggs can serve as a growth medium to deadly microorganisms (8). Bacterial growth can be prevented with the use of methylene blue.
Embryo processing has a significant role with regards to the experimental outcome as healthy and well-developed embryos will render more accurate and reliable results, thus improving the strength of the study. Including unfertilised eggs or premature dead embryos in a cohort of tested embryos can lead to unprecise results as unfertilised eggs will increase the death rate following a screening assay. Therefore, early sorting of embryos should be performed not only to prevent batch contamination but also to exclude any irrelevant samples from the experiment. (3)
Egg sorting: algorithms and intelligent devices
Although many morphological features can be used to sort fertilised and non-fertilised zebrafish eggs, manual sorting remains an unprecise task prone to errors. Therefore, several algorithms have been developed for egg sorting and classification and have been implemented in various automated devices (7). Not only does automation increase the tasks speed but also accuracy which will in the end improve the strength of the results, by reducing errors and increasing the number of samples which can be analysed and included in the experiment.
Automation relies (mostly) on machine learning technologies. High-throughput screening and artificial intelligence are nowadays at the heart of zebrafish experimentation and are widely recognised as essential for screening assays. If you would like to learn more on how AI can be used within zebrafish experimentation, have a look at our previous article: Artificial intelligence for zebrafish egg sorting.
The EggSorter by Bionomous does use these technologies to automatically screen, sort and dispense zebrafish eggs based on different classification criteria, such as the fertilisation status. Thus, making possible the automation of this important step when working with zebrafish embryos.
References
(1) PubMed.gov. Retrieved October 24th, 2020. https://pubmed.ncbi.nlm.nih.gov/?term=zebrafish
(2) Kari, G., Rodeck, U., & Dicker, A. P. (2007). Zebrafish: an emerging model system for human disease and drug discovery. Clinical pharmacology and therapeutics, 82(1), 70–80. https://doi.org/10.1038/sj.clpt.6100223
(3) Meyers, J. R. (2018). Zebrafish: Development of a vertebrate model organism. Current Protocols Essential Laboratory Techniques, e19. doi: 10.1002/cpet.19
(4) Darrow, K. O., & Harris, W. A. (2004). Characterization and development of courtship in zebrafish, Danio rerio. Zebrafish, 1(1), 40–45. https://doi.org/10.1089/154585404774101662
(5) Nasiadka, A., & Clark, M. D. (2012). Zebrafish breeding in the laboratory environment. ILAR journal, 53(2), 161–168. https://doi.org/10.1093/ilar.53.2.161
(6) Adatto, I., Lawrence, C., Thompson, M., & Zon, L. I. (2011). A new system for the rapid collection of large numbers of developmentally staged zebrafish embryos. PloS one, 6(6), e21715. https://doi.org/10.1371/journal.pone.0021715
(7) Chen, Q., Di, Z., García Roger, E. M., Li, H., Richmond, P., & Roehner, B. M. (2020). Magnitude and significance of the peak of early embryonic mortality. Journal of biological physics, 46(3), 233–251. https://doi.org/10.1007/s10867-020-09555-4
(8) Woynarovich, E., Horváth, L. (1980) The artificial propagation of warm-water fin-fishes – a manual for extension. FAO Fish.Tecg.Pap., (201):183 p. http://www.fao.org/3/ac742e/AC742E05.htm
(9) Neukum, A., Bartschat, A., Breitwieser, H., Strähle, U., Dickmeis, T., & Pylatiuk, C. (2019). Automated Classification of Fertilized Zebrafish Embryos. Zebrafish, 16(3), 326–328. https://doi.org/10.1089/zeb.2019.1728
(10) FishForPharma. Retrieved October 31st, 2020. https://www.fishforpharma.com/why-zebrafish-17