Halloween is right around the corner and you will soon be seeing various spooky creatures parading down the streets: ghosts, wizards and zombies which might even be missing a limb. While ghosts and wizards are usually classified as magical creatures, zombies have something in them which make them slightly more real. A zombie is an animated corpse which has been brought back to life and although resurrection remains completely unreal, regenerative medicine has been working on replacing and regenerating defective cells, tissues and organs with the goal of restoring normal function.
Some animals are capable of independently regenerating various organs. Have you ever tried to catch a lizard by its tail and end up with a piece of tail in your hand while the tailless creature runs away? How does its tail grow back? All of these questions have been triggering scientists over the past couple of centuries. To get a better insight in regeneration and limb regrowth, zebrafish have become a popular model organism as they are capable of regenerating a fully functional caudal fin as well as other more complex internal organs. To know more about regeneration in zebrafish and humans, read on!
Danio rerio, an ideal model organism for organ regeneration
Zebrafish are among the few vertebrates to be able to regenerate complex external and internal organs. Indeed, following surgical, chemical or genetic ablation, zebrafish can regrow functional tissue in the heart, liver, kidneys, brain, eye and caudal fin1. Due to the external location of fins on the fish’s body, fin regeneration was the first organ to be studied to understand tissue regeneration. In 1786, a French scientist made the first observations of fin regeneration on a gold fish and stated “I remarked that the fins were renewed generally sooner or later, according as they were more or less useful to the animal.” (Broussonet)2. Since then, fish tails have become a popular model system to investigate cellular and molecular mechanisms underlying organ restoration in vertebrates2.

Zebrafish are a powerful model to investigate appendage regeneration as they hold many advantages4. First of all, fins are accessible and can easily be amputated without causing any major detrimental effects to the fish in laboratory conditions. Secondly, fin regeneration is fast and can easily be monitored in vivo using live imaging techniques thanks to the transparency and minimal thickness of the fin. Within 2-3 weeks, zebrafish are capable of restoring a fully functional organ which reaches the same size, pattern and tissue organization as the original appendage.
The caudal fin has bi-lobed anatomy and is composed of several rays which can regenerate independently from one another. Therefore, it is an ideal organ to observe differential growth rates among the medial-lateral axis and to perform experiments with multiple experimental replicates within the same appendage2. Finally, the zebrafish’s genome is well known and therefore multiple genetic tools are available for genetic manipulations.
Caudal fin anatomy
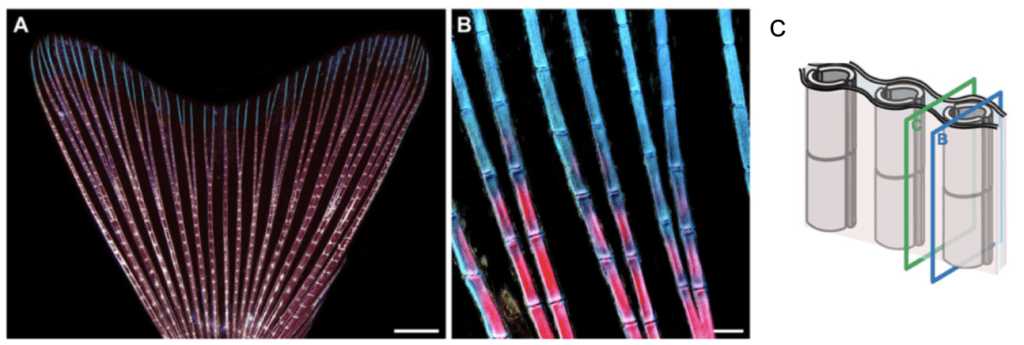
Figure 1: Caudal fin anatomy2. (A) The caudal fin is composed of 16-18 bifurcated rays and displays a bi-lobed morphology. Rays are made up of multiple segments which are interconnected by intersegmental joints. The proximal portion of the rays is stained in pink due to the calcified bone matrix whereas the distal portion of the rays is stained in blue due to the collagen bone matrix. Ligaments are present at the borders of each segment and are displayed in white. (B) High magnification of the caudal fin demonstrating the proximal-distal gradient of calcification in the fin rays. (C)Two hemi-rays composed of a concave bone each constitute each ray. B and C planes demonstrate respectively a transverse section of the bony rays and the inter-ray mesenchymal tissue.
The zebrafish caudal fin is a non-muscularised dermal fold which originates from the ventral side of the larval fin and is composed of 16-18 bony rays which can be bifurcated2. Caudal fin rays are segmented and articulated between each other by inter-segmental joints, providing flexibility to the fin5. Rays are made up of a pair of concave bones each which together appear as parallel rods below the multi-layered epidermis of the fin. The collagenous bone matrix of the fin rays provides robustness to the thin. However, only the proximal portion of the rays possesses an additional calcified bone matrix. The distal portion of the rays on the other hand is composed of brush-like bundles of thin and flexible spicules which provide optimal architectural function to the organ2. The space between two concave bones is filled with a dense connective tissue composed of interconnected fibroblasts which embeds nerves and arteries. The space between two rays is filled with a loose mesenchymal tissue2. In sum, all of these distinct tissues represent a wide variety of cells which need to be regenerated co-ordinately to restore proper organ pattern and function.
Caudal fin regeneration
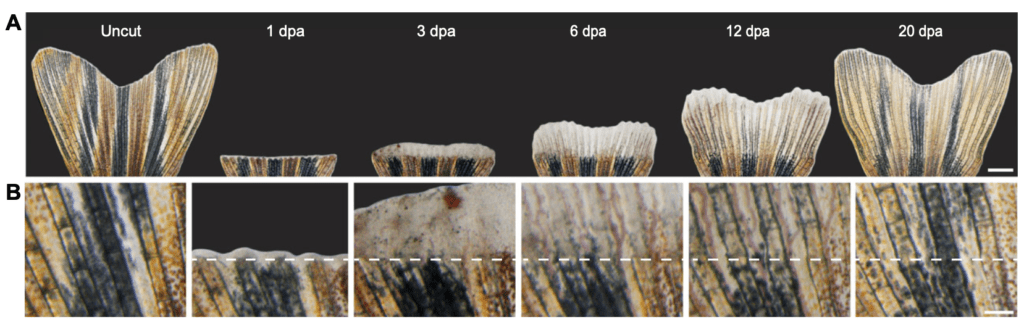
Figure 3: Caudal fin regeneration time line.2 (A) Zebrafish can regenerate a fully functional caudal fin in 3 weeks. (B) High magnification view of the caudal fin regeneration above the amputation line.
Two main events follow caudal fin amputation. First, epithelial cells migrate to the amputation site and form a regeneration epidermis to cover and protect the wound. This event coincides with the migration of undifferentiated cells to the distal end of the fin. Migrating cells include de-differentiated fibroblasts and osteoblasts and form a regeneration structure, namely the blastema. Such cells are capable of dedifferentiating, proliferating and re-differentiating into the majority of cells necessary to restore fin shape and function4.
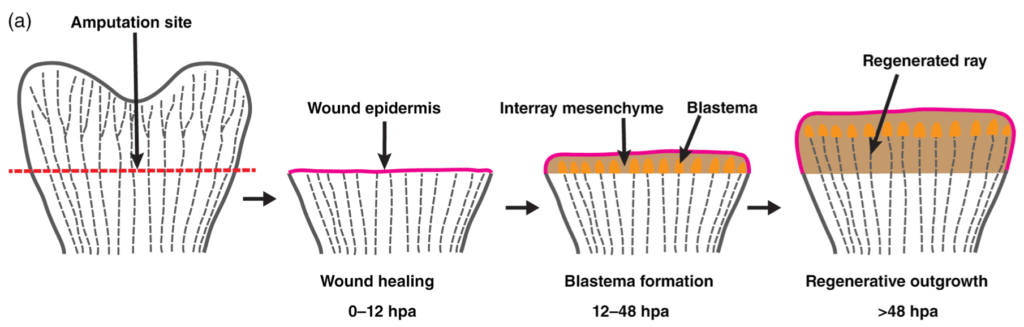
Figure 4: Caudal fin regeneration.4 Fin regeneration begins with the formation of a regeneration epidermis and is followed by migration and proliferation of de-differentiated cells at the distal end of the caudal fin which form the blastema.
In order to regenerate an identical fin to the original, cells must follow molecular cues. Signalling pathways and molecular gradients enable fin restoration and growth with the appropriate dimensions and within the correct axis of the organism. Studies have demonstrated that cells within each fin ray possess their own positional information6. Therefore, medial rays will grow until they reach an intrinsically defined length, which by definition is shorter than the lateral rays. In addition, fin ray regeneration period and rate depend on the amputation site. Regeneration rate and period will respectively be faster and longer when the amputation site is proximal to the fin stump compared to a distal amputation7.
Why can't humans regenerate limbs?
Humans are capable of regenerating some tissues up to a certain extent. Following surgical resection of the liver, the organ can regrow but will not reach the same functional capacities. The heart, skin, gut and bones also regenerate somehow in the sense that tissue is slowly replaced overtime. However, humans and other vertebrate animals cannot regrow limbs. Although scientists are working hard to uncover the mechanisms behind limb regeneration in zebrafish or salamanders, reasons why humans don’t possess this capacity remain unclear. Several theories are out there and reside in tissue complexity, limited stem cell pool, inactivated cellular machinery or simply adaptation8. Indeed, a human limb is much more complex and is made up of many more tissues than a salamander limb or zebrafish caudal fin. Mistakes during regeneration are highly likely and could lead to much more severe consequences than the missing limb itself. For instance, excessive cell proliferation could rapidly get out of control and end up in tumour growth. Some theories suggest that regeneration in fish is possible thanks to the reactivation of certain genetic programs via epigenetic modifications2. Therefore, during evolution, vertebrate mammals may have lost the ability to reactivate certain genetic programs and form scar tissue instead which will protect the wound but inhibit regeneration.
To conclude, although regenerative medicine has made huge progress over the past decade, humans cannot regrow a functional limb following amputation. On the other hand, zebrafish can regrow many body parts multiple times, reaching the exact same morphology and functional capacities. Additionally, zebrafish are far more real than zombies or any other magical creature. Does that not make them slightly more spooky?
References
- Marques, I. J., Lupi, E. & Mercader, N. Model systems for regeneration: zebrafish. Development 146, dev167692 (2019).
- Pfefferli, C. & Jaźwińska, A. The art of fin regeneration in zebrafish: The Art of Fin Regeneration. Regeneration 2, 72–83 (2015).
- Pierre Marie Auguste Broussonet. Wikipédia (2020).
- Sehring, I. M. & Weidinger, G. Recent advancements in understanding fin regeneration in zebrafish. WIREs Dev. Biol. 9, (2020).
- Borday, V. R. et al. evx1 transcription in bony fin rays segment boundaries leads to a reiterated pattern during zebrafish fin development and regeneration. 8 (2001).
- Shibata, E., Liu, Z., Kawasaki, T., Sakai, N. & Kawakami, A. Robust and local positional information within a fin ray directs fin length during zebrafish regeneration. Dev. Growth Differ. 60, 354–364 (2018).
- Uemoto, T., Abe, G. & Tamura, K. Regrowth of zebrafish caudal fin regeneration is determined by the amputated length. Sci. Rep. 10, 649 (2020).
- Westreich, S. Why Can’t We Regrow Limbs? Sharing Science https://medium.com/a-microbiome-scientist-at-large/why-cant-we-regrow-limbs-32ba3c42e245 (2020)



