For the third week of our special Women’s month, we are going to present to you Dr. Sharon Amacher, an American scientist who dedicates her research to understanding muscle development, patterning and disease.
Biography
Sharon Amacher completed her entire academic studies in the United States. She first attended the University in California Berkeley to obtain a Bachelors Degree in Physiology. She then continued her studies at the University of Washington in Seattle where she obtained a PhD in biochemistry in 1993.
After her PhD, she worked at the University of Oregon as a Postdoctoral Fellow (1). Her first independent position was in the Department of Molecular and Cell Biology at the University of California Berkeley where she ascended through the ranks to Full Professor. In 2012, she moved her laboratory to the Department of Molecular Genetics at the Ohio State University. She and her team study muscle biology using zebrafish as a model organism (2).

Her contribution to zebrafish research
In 2008, Amacher and her collaborators at Sangamo pioneered the use of zinc finger nucleases (ZFNs) to mutate genes in zebrafish. With this method, they were one of two collaborative teams (the other being Nathan Lawson and Scot Wolfe) to publish back-to-back papers showing that ZFNs can generate targeted mutations that are stably inherited through the germline.
Her article “Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases” has been cited more than thousand times (according to google scholar). This article describes the method they used to induce targeted double-strand breaks in the genome of zebrafish to generate mutagenesis (3). The success of this study led to a major step in genetic engineering and reverse genetics in this model.
Zinc finger nucleases are designed nucleases that induce double-strand breaks in a targeted place in the genome. They are composed of two domains: a DNA-binding domain made up of zinc finger modules and an enzyme (nuclease) that cleaves the DNA (4).
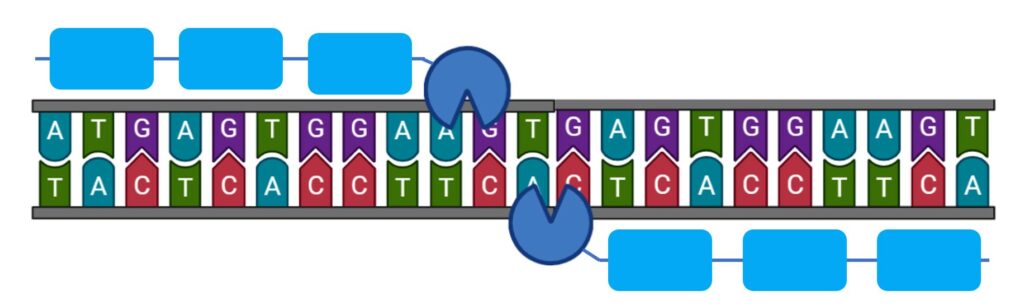
Her research on genome editing allowed for the expansion of available zebrafish mutants and foreshadowed the next wave of gene targeting methods like CRISPR/Cas9 (10). Being able to create targeted gene mutation is of great importance in the understanding of biological mechanisms and to study genetic diseases.
In addition to her contribution toward gene targeting technology, she continues studying vertebrate embryo development by using zebrafish as a model. More specifically, the research of her team focuses on muscle development and patterning (1,5,6).
Sharon’s group published the first segmentation clock reporter with single-cell resolution (11, 12). Her group was the first to carefully show that adult zebrafish muscle contains muscle stem cells responsible for skeletal muscle growth and repair that are strikingly similar to muscle stem cells in other vertebrates (8). They have also collaborated with others to study muscle-specific splicing regulators (9), muscle cell migration (13), muscle cell fusion (14), and other muscle diseases like distal arthrogryposis (15) and rhabdomyosarcoma (16).
Conclusion
Sharon’s areas of expertise (vertebrate development, muscle development, oscillatory gene expression and RNA biology (6)) have allowed her to make significant contributions to the advancement of research on biological patterning mechanisms and muscle degenerative diseases such as muscular dystrophy.
Her work has also contributed to creating zebrafish models of human disease (9) and helped to establish the efficiency and reliability of a new method in genetic engineering.
References
- Sharon L. Amacher | Ohio State University – Nationwide Children’s Hospital Center for Muscle Health and Neuromuscular Disorders [Internet]. [cited 2023 Mar 13]. Available from: https://osuchildrensmusclegroup.org/faculty-research/sharon-l-amacher/
- Lab Members | Department of Molecular Genetics [Internet]. [cited 2023 Mar 14]. Available from: https://molgen.osu.edu/amacher/people
- Doyon Y, McCammon JM, Miller JC, Faraji F, Ngo C, Katibah GE, et al. Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nat Biotechnol. 2008 Jun;26(6):702–8.
- Jo YI, Kim H, Ramakrishna S. Recent developments and clinical studies utilizing engineered zinc finger nuclease technology. Cell Mol Life Sci. 2015 Oct;72(20):3819–30.
- Amacher Laboratory | Department of Molecular Genetics [Internet]. [cited 2023 Mar 13]. Available from: https://molgen.osu.edu/amacher
- Sharon Amacher | Department of Molecular Genetics [Internet]. [cited 2023 Mar 13]. Available from: https://molgen.osu.edu/people/amacher.6
- Current Research Projects | Department of Molecular Genetics [Internet]. [cited 2023 Mar 14]. Available from: https://molgen.osu.edu/amacher/research
- Berberoglu MA, Gallagher TL, Morrow ZT, Talbot JC, Hromowyk KJ, Tenente IM, et al. Satellite-like cells contribute to pax7-dependent skeletal muscle repair in adult zebrafish. Dev Biol. 2017 Apr;424(2):162–80.
- Gallagher TL, Arribere JA, Geurts PA, Exner CRT, McDonald KL, Dill KK, et al. Rbfox-regulated alternative splicing is critical for zebrafish cardiac and skeletal muscle functions. Dev Biol. 2011 Nov;359(2):251–61.
- Talbot JC, Amacher SL. A Streamlined CRISPR Pipeline to Reliably Generate Zebrafish Frameshifting Alleles. Zebrafish. 2014 Dec;11(6):583–5
- Delaune EA, François P, Shih NP, Amacher SL. Single-Cell-Resolution Imaging of the Impact of Notch Signaling and Mitosis on Segmentation Clock Dynamics. Dev Cell. 2012 Nov;23(5):995–1005.
- Shih NP, François P, Delaune EA, Amacher SL. Dynamics of the slowing segmentation clock reveal alternating two-segment periodicity. Development. 2015 May 15;142(10):1785–93.
- Talbot JC, Teets EM, Ratnayake D, Duy PQ, Currie PD, Amacher SL. Muscle precursor cell movements in zebrafish are dynamic and require six- family genes. Development. 2019 Jan 1;dev.171421.
- Hromowyk KJ, Talbot JC, Martin BL, Janssen PML, Amacher SL. Cell fusion is differentially regulated in zebrafish post-embryonic slow and fast muscle. Dev Biol. 2020 Jun;462(1):85–100.
- Chong JX, Talbot JC, Teets EM, Previs S, Martin BL, Shively KM, et al. Mutations in MYLPF Cause a Novel Segmental Amyoplasia that Manifests as Distal Arthrogryposis. Am J Hum Genet. 2020 Aug;107(2):293–310.
- Hsu JY, Danis EP, Nance S, O’Brien JH, Gustafson AL, Wessells VM, et al. SIX1 reprograms myogenic transcription factors to maintain the rhabdomyosarcoma undifferentiated state. Cell Rep. 2022 Feb;38(5):110323.
First image : NIH funding to Amacher lab! | Department of Molecular Genetics [Internet]. [cited 2023 Mar 14]. Available from: https://molgen.osu.edu/news/nih-funding-amacher-lab
Read and approved by Sharon Amacher



