In this new series of articles, we will review some of the key scientific publications of 2020 which included zebrafish in their studies. This first review will cover an article published in Clinical Cancer Research on March 15th 2020: Deubiquitinase Activity Profiling Identifies UCHL1 as a Candidate Oncoprotein that Promotes TGFβ-induced breast cancer metastasis1.
In this article, the authors have focused their research on a subset of proteins involved in the tumour cell intracellular degradation pathway as a potential treatment for breast cancer. To get a full comprehensive overview of the content of this article, we have first briefly described some common breast cancer subtypes and important terms such as metastasis, epithelial mesenchymal transition and intracellular pathway. Then we have pointed out the findings and outcomes of the study as well as the advantages of using zebrafish xenograft models for cancer research experiments.
Studying breast cancer
Breast cancer is the leading type of cancer in women and the second cause of death in the USA. 1 out of 8 women will develop breast cancer during their lifetime. However, when diagnosed early the prognosis is often favourable.
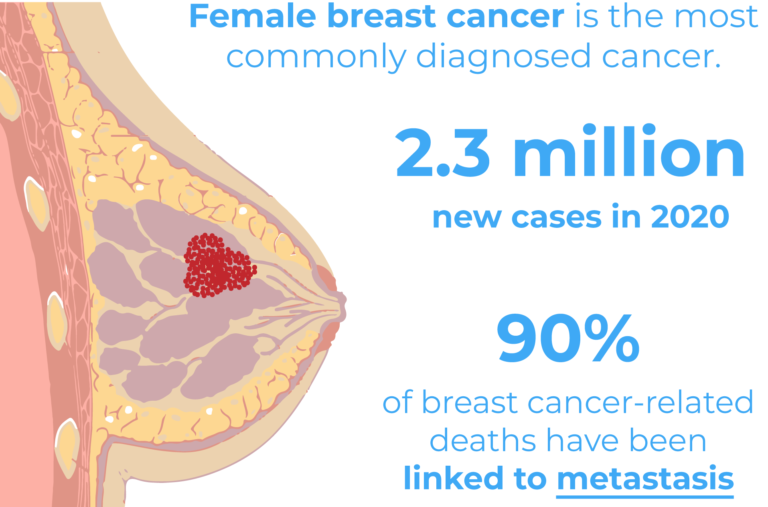
There are several subtypes of breast cancer with different metastatic potentials, aggressiveness and severities. Tumour size, nodal involvement, grade, lymphovascular invasion, estrogen receptor (ER) and human epidermal growth factor receptor (HER2) all affect the disease severity2. Typically, HER2 and ER expression status have been associated with more severe subtypes of tumours and with an increased risk of metastasis2. The triple-negative breast cancer (TNBC) subtype however, has been identified as the most aggressive and challenging subtype of breast cancer due to the low response rate to currently available treatments. TNBC tumours lack estrogen and progesterone receptors as well as HER23, which are typically targeted by anti-cancer therapies.
The lack of clinically meaningful targets for this type of cancer has motivated researchers to uncover novel targets for new effective drugs1.
Targeting metastasis
90% of breast cancer-related deaths have been linked to metastasis, a process during which cancer cells detach from the original tumour and spread to different locations through the blood and lymphatic system and eventually give rise to new tumours.
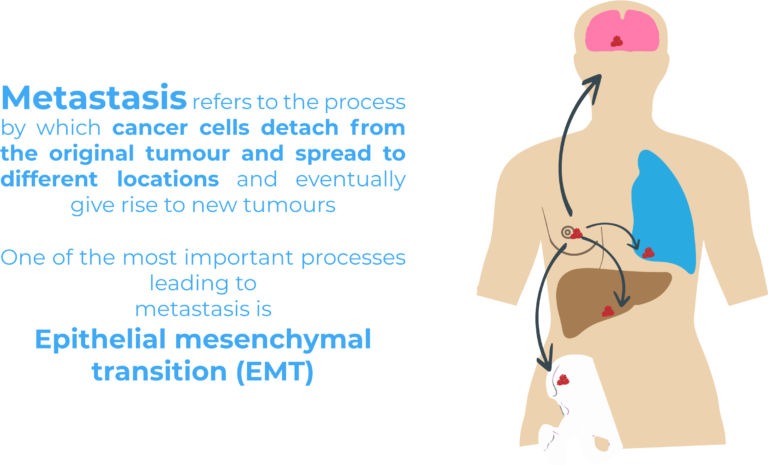
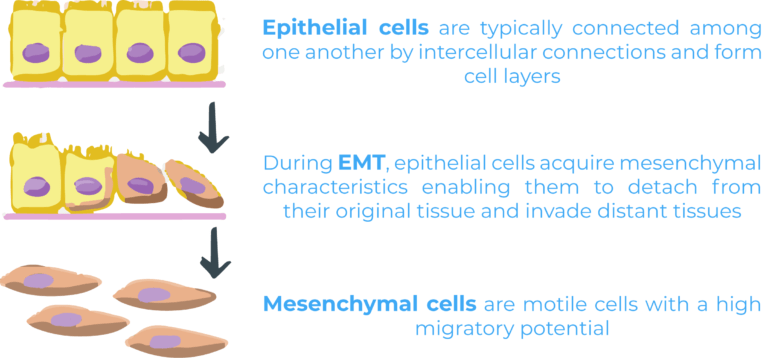
Epithelial mesenchymal transition (EMT) is one of the most important processes leading to metastasis. During EMT, epithelial cells acquire mesenchymal characteristics enabling them to detach from their original tissue and invade distant tissues. Epithelial and mesenchymal cells are two very different cell populations. Epithelial cells are typically connected among one another by intercellular connections and form cell layers making up tissues such as the skin. On the other hand, mesenchymal cells are motile cells with a high migratory potential. While EMT occurs naturally during embryogenesis and wound healing, it has also been linked to malignant progression4.
Several signals converge to activate EMT through various pathways. A typical cellular pathway involves binding of a molecule (eg. a molecular signal such as a cytokine) to a corresponding receptor, recruitment of intracellular proteins and activation of transcription factors which will interact with the DNA and modulate gene transcription. Many cellular pathways are involved in EMT, transforming growth factor-β (TGFβ) pathway being a crucial one.
TGFb is a secreted cytokine which plays a crucial role in EMT activation in neoplastic tissues and therefore is an interesting pathway to target in novel therapies. The TGFβ pathway is tightly regulated by ubiquitination – a key regulatory mechanism in cell biology – during which ubiquitin proteins are added to substrate proteins to target them for degradation. Ubiquitin E3 ligase enzymes and deubiquitinases (DUB) mediate respectively ubiquitination (addition of ubiquitin proteins) and deubiquitination (removing ubiquitin proteins). Emerging studies demonstrate that DUBs have an important role in cancer development and progression.
What have they found in this study?
The authors of this study decided to analyse the activity of DUBs in human breast cancer cell lines and patient tumour tissues. This was done by assessing DUB activity profiles using so called DUB activity-based probes in different breast cancer subtypes. They were able to point out ubiquitin carboxy-terminal hydrolase L1 (UCHL1) with an increased activity in TNBC cells lines and ER negative patient tumour tissues.
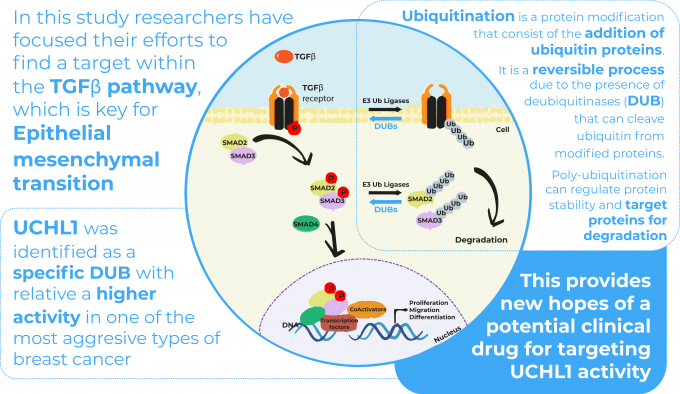
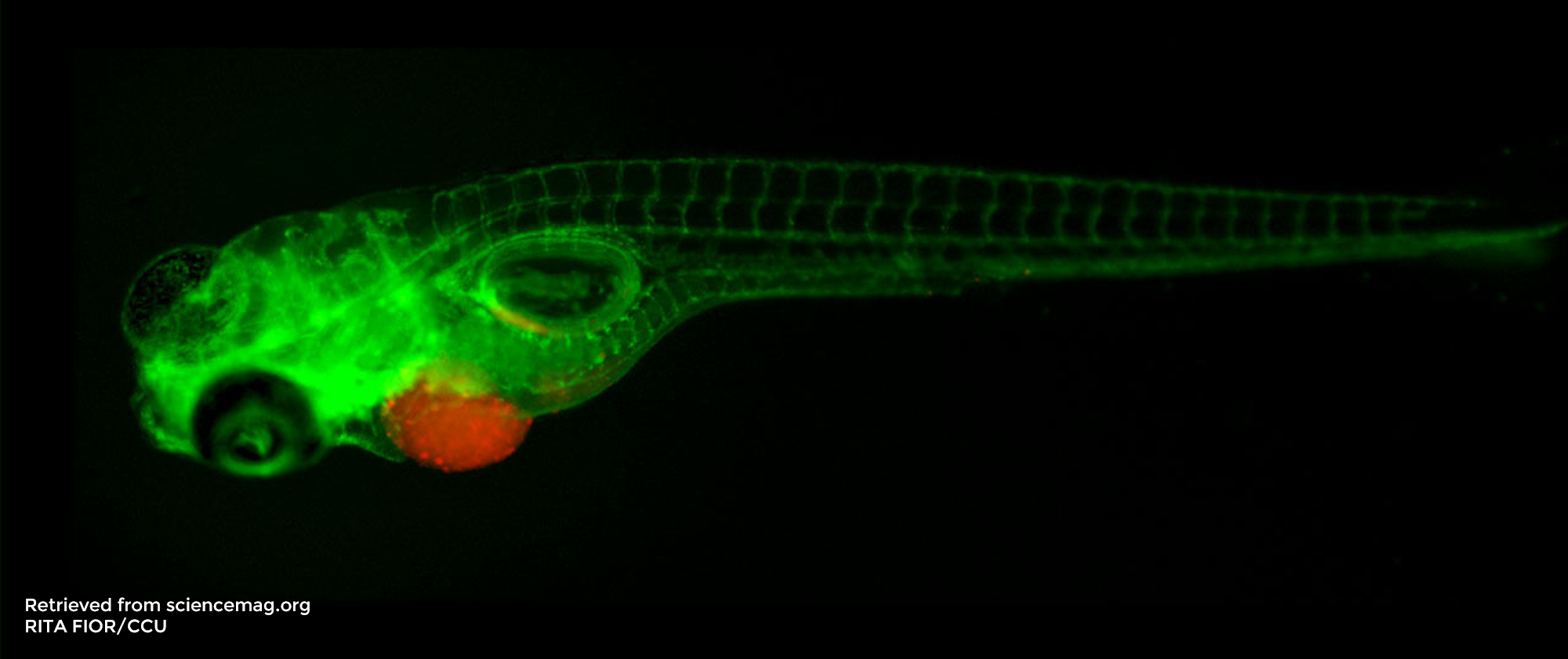
Once UCHL1 had been identified as an important DUB involved in breast cancer, they wanted to evaluate whether UCHL1 interacted with the TGFβ signalling pathway, as this is a key pathway in metastasis and tumour malignancy. Among different experiments (see full article), they tested the importance of UCHL1 in TGFβ-induced breast cancer metastasis in vivo using a zebrafish embryo xenograft model in the context of several extravasation assays. A xenograft model is an animal model in which cells from another species are injected to study their behaviour in vivo.
Zebrafish as hosts for xenograft cell lines
Zebrafish embryos are excellent hosts for xenografts as their immune system is not yet developed and therefore xenografted (human) cancer cells are not rejected. They are efficient, reliable and low-cost models, which allow one to obtain fast results in the context of epigenetic or pharmacological studies5. Moreover, their high genetic homology to humans and the transparency of the zebrafish embryos have made zebrafish popular vertebrate models in many research fields.
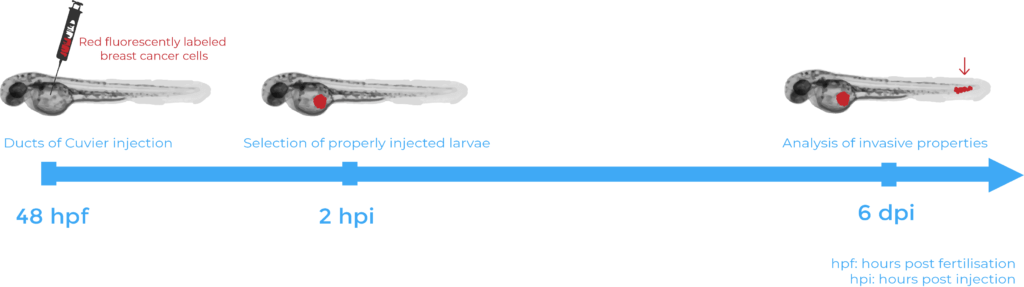
Extravasation assays required injection under the microscope of chemically or genetically fluorescent labelled human breast cancer cells into specific transgenic zebrafish line embryos, known to express fluorescent blood vessels5. The cells were injected into the Ducts of Cuvier (also known as the perivitelline space) 48 hours after fertilisation. 6 days after injection, the zebrafish were fixed with 4% formaldehyde and observed under an inverted confocal microscope. Several hypotheses were validated using such assays. First, the injection of human breast cancer cells with a high UCHL1 expression into zebrafish embryos demonstrated that metastasis is linked to high cellular levels of UCHL1. Then, the lack of cell migration following inhibition of the TGFβ receptors in the injected human breast cancer cells validated that UCHL1-induced metastasis relies on its ability to potentiate TGFβ signalling. Finally, selective inhibition of UCHL1 provided a final piece of evidence that UCHL1 is required to promote breast cancer migration and extravasation. Indeed, cells treated with the inhibitor showed less cell extravasation in the zebrafish embryos.
Conclusion
In conclusion, UCHL1 was identified as a deubiquitinase with relative higher activity in the TNBC subtype. Although zebrafish were not the only model utilised in the context of this study, the zebrafish xenograft model proved to be an interesting research model to observe several cancer mechanisms and validate multiple hypotheses. They allowed to observe in vivo how UCHL1 can promote breast cancer migration and extravasation. Moreover, UCHL1 was identified as a modulator of the TGFβ pathway and TGFβ-induced breast cancer cell migration and extravasation. This outcome provides new hopes of a potential clinical drug for targeting UCHL1 activity in the treatment of aggressive breast cancer and other UCHL1 overactive cancers.
References
- Liu, S. et al. Deubiquitinase Activity Profiling Identifies UCHL1 as a Candidate Oncoprotein That Promotes TGFβ-Induced Breast Cancer Metastasis. Clin. Cancer Res. 26, 1460–1473 (2020).
- Kennecke, H. et al. Metastatic Behavior of Breast Cancer Subtypes. J. Clin. Oncol. 28, 3271–3277 (2010).
- Foulkes, W. D. & Reis-Filho, J. S. Triple-Negative Breast Cancer. N. Engl. J. Med. 11 (2010).
- Xu, J., Lamouille, S. & Derynck, R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 19, 156–172 (2009).
- Ren, J., Liu, S., Cui, C. & ten Dijke, P. Invasive Behavior of Human Breast Cancer Cells in Embryonic Zebrafish. J. Vis. Exp. 55459 (2017) doi:10.3791/55459.