As spring fades into summer and nature bursts into full bloom, it is a fitting time to reflect on scientific approaches that promote innovation while advancing more ethical and sustainable research practices. This past spring was filled with events and initiatives that energized the research community and accelerated the development of forward-thinking solutions. Among them, New Approach Methodologies (NAMs) are emerging as powerful alternatives to traditional animal testing.
What are NAMs exactly? How can they effectively be used in research? And what is the vision of regulatory bodies on that matter? This month’s article aims to introduce NAMs different categories, show examples compatible with Bionomous’ EggSorter and explore how they could reshape the future of research and safety assessment.
NAMs, NATs & regulations
In recent years, there has been increasing awareness that traditional animal models often fall short in predicting how a drug will behave in humans. Indeed, more than 90% of drug candidates that pass animal tests fail in human clinical trials, mostly due to unexpected safety and/or efficacy issues (1). In addition to ethical concerns, this contributes to longer development timelines and substantial financial costs. As a result, there is growing interest within the scientific community in developing more physiologically relevant, ethical, and cost-effective approaches to preclinical safety studies, leading to the emergence of New Approach Methodologies (NAMs).
NAMs represent a broad and evolving set of tools designed to improve the way we assess chemical safety, drug efficacy, and biological responses while reducing or eliminating the need for traditional animal testing. The term “NAM” was officially introduced during a 2016 workshop organized by the European Chemicals Agency (ECHA) in Helsinki (2), though its roots lie in earlier efforts to advance ethical and scientifically robust alternatives. Depending on the context, NAMs may stand for Novel Alternative Methods, Non-Animal Methods, or New Alternative Methods.
NAMs are defined as any technology, methodology, or approach that can provide data relevant to hazard identification or risk assessment without relying on the use of live, intact animals. This includes a wide array of systems, from advanced in vitro human-derived models to in silico computational tools and predictive technologies, in chemico molecular interaction studies (3) and other systems like ex vivo tissues or non-sentient organisms, also called Non-Protected Life Stages, NPLS (e.g., fish eggs).
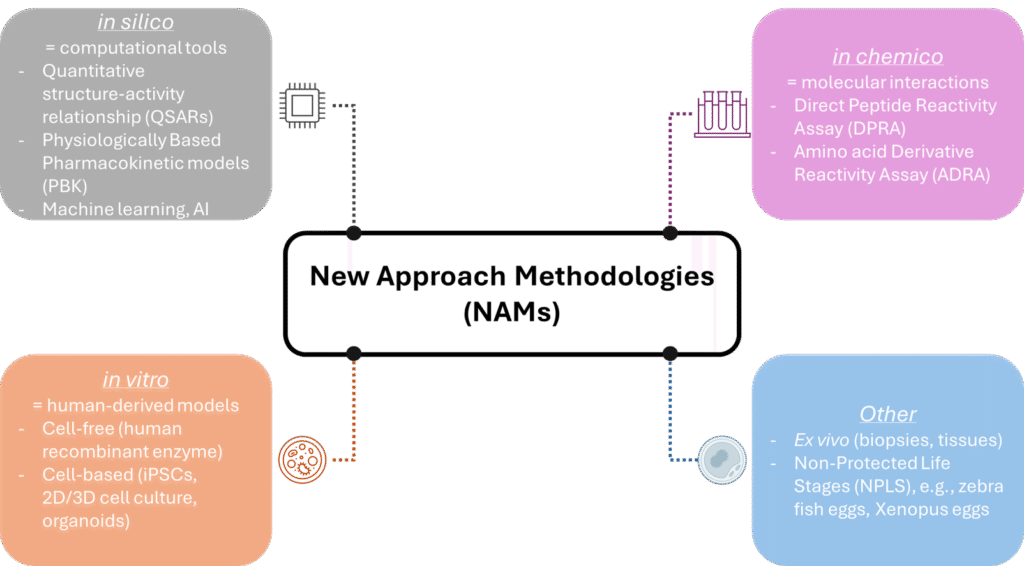
Closely related is the concept of Non-Animal Technologies (NATs), referring to fully animal-free tools and platforms, making them a subset of NAMs. Both NAMs and NATs align with the 3Rs principles (Replacement, Reduction, Refinement of animal use) while also offering distinct advantages in cost-efficiency, time savings, ethical acceptability, and human relevance. By leveraging modern technologies, they enhance our ability to model complex human biology more precisely than conventional animal studies.
While NAMs offer significant advantages, their uptake in regulatory toxicology has been slower than projected. Although specific technical challenges remain, one of the major reasons for this slow uptake appears to be the gap between current, animal-centred legislations and NAMs approaches (4). Indeed, as animal tests are now standardized, widely accepted and legally clear, it is difficult to directly translate them to NAMs, which imply a fundamentally different approach.
Regulatory frameworks need to evolve to explicitly recognize and accommodate their use in safety assessment, and encouraging progress is already underway. This April, the U.S. Food and Drug Administration (FDA) released their new plan to phase out animal testing in the context of monoclonal antibodies, paving the way for a shift to NAMs as the norm for preclinical testing (1, 5). In Europe, ECHA and the European Commission just completed their workshop series “roadmap to phase out animal testing for chemical safety assessments” this month, aiming to integrate New Approach Methodologies (NAMs) across EU legislation (6).
Change is underway, driven by the combined efforts of researchers, industry, and regulators working toward more ethical and effective testing methods. As a result, the field of NAMs is expanding, offering a wide range of approaches, each with its own strengths and limitations depending on the context of use. To better understand this evolving landscape, the next section explores promising examples, such as fish eggs and organoids, and how they are helping to shape the future of non-animal testing.
Zebrafish eggs

Zebrafish or Danio rerio, are small teleost fish native to South and Southeast Asia, commonly found in clear, shallow waters such as river basins in India. Their name comes from the distinct horizontal stripes on their body, making them easy to recognize.
Since their first use in research in the 1930s, zebrafish eggs popularity has kept increasing, making them one of nowadays’ most promising NAMs. This adoption is mainly due to their prolific breeding (100+ eggs) and rapid development (36 hpf), as well as their human relevance (70% genetic similarity) and their regenerative capabilities. More information on our zebrafish article.
Consequently, there has been an ongoing effort to develop protocols and investigate the relevance of such models as an alternative to standard mammalians models, especially in Ecotoxicological testing and Developmental & Reproductive Toxicity.
Zebrafish eggs have been used as models for endocrine-disrupting chemical (EDC) testing in order to perform early-stage environmental assessments (7).
The potential negative effects of agrochemicals like insecticides, herbicides and fungicides on the early-stage development can also be assessed using zebrafish eggs. By following the Zebrafish Embryo Developmental Toxicity Assessment (ZEDTA), researchers found promising but variable results compared to mammalians models (8).
Zebrafish eggs are also be used as a potential NAM to assess the teratogenicity of certain chemicals by evaluating morphological assessments like malformations, and lethality (9). Some studies also compare the performance of different NAMs with respect to their successful classification of both known teratogenic and non-teratogenic chemicals (sensitivity & specificity). While Zebrafish Embryotoxicity Tests (ZET) showed good results, a combined NAMs approach could also be of interest. Indeed, by combining in silico (e.g., QSAR – DART), in vitro cell-based (e.g., devTOX quickPredictTM) and ZET methods, a “2 out of 3” selection rule can lead to even more robust results (10).
Several official regulations exist for the use of zebrafish embryos in acute and general toxicity testing, such as OECD TG 236 (Fish Embryo Acute Toxicity), TG 210 (Early life toxicity) or TG 212 (short term toxicity embryo). These tests are offered as screening services by many service providers (11, 12, 13, 14, 15, 16), however a more robust regulatory framework is needed for them to offer real alternatives to standard mammalians models.
Medaka eggs
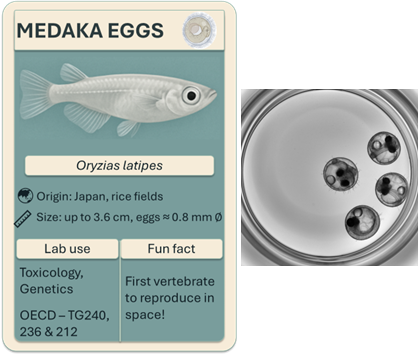
Medaka, or Oryzias latipes, are small teleost fish native to Japan’s rice paddies. Originally kept as pets in Japan since the 17th century, medaka have become increasingly valuable in research and were even the first vertebrates to mate in space! They are an interesting and versatile organism in research mostly because of their total transparency, their compact and well characterized genome and their exceptional resilience (e.g., 0-42°C tolerance).
As medaka eggs are highly responsive to toxicant exposure during early development, they have been used in Developmental Toxicity studies as a potential alternative to conventional animal testing.
In recent studies, medaka eggs have been used as a model to validate the impact of solvents typically used in toxicity studies, like dimethyl sulfoxide (DMSO). New protocols and labware are increasingly being developed to account for high-throughput studies (17), which would contribute to accelerating the global transition to NAMs.
Medaka eggs have also been used to study human-like metabolism, as an alternative to human volunteers or standard animal models. For example, studies have emerged in antidoping science, by evaluating the impact of steroids exposure on medaka eggs (18). By assessing malformations (e.g., heart, tail) according to FET protocol as well as heartbeat and metabolites, the researchers could demonstrate the relevance of this model to study human-like metabolism.
With today’s prevalence of skin cancer, there is a need to develop more economical, ethical and suitable platforms for the screening of potential protective compounds. The suitability of medaka eggs to studying the harmful effects associated to exposure to sunlight has been investigated and compared to other existing models such as 2D and 3D human cell cultures (19). By evaluating both UVB’s DNA damage and UVA’s Reactive Oxygen Species (ROS), the study revealed that medaka eggs constitute a good model, as they exhibit real skin protection mechanisms that the oversimplistic cell culture models are lacking. Thus, medaka eggs show potential to be used as a suitable screening platform for molecules with counteracting capacities against sunlight damage.
The OECD currently provides either endocrine-specific assessment guidelines in adult medaka (TG 229 and TG 240) or general toxicity protocols for fish eggs, including medaka (TG 210 and TG 212), framing the use of medaka eggs as a NAM. Despite the growing interest, regulatory adoption is limited by the lack of standardized protocols, species-specific guidelines, and inter-laboratory validation. Broader validation and regulatory updates are needed to support their integration.
Killifish eggs

African turquoise killifish, also known as Nothobranchius furzeri, are freshwater colourful fish originating from seasonal ponds in Mozambique and Zimbabwe. Since the early 2000s, the eggs of N. furzeri have been used in research, especially due to their transparent and numerous eggs (50+), their fast aging (lifespan < 6 months) and their adaptability to extreme environments, most notably droughts during which the eggs can survive in dry mud in a state of suspended development, referred to as diapause (20).
These unique features make killifish eggs and larvae a very appealing model in studies focusing on age-related diseases and degenerative mechanisms as well as in ecotoxicological research.
Due to their very short lifespan, N. furzeri is an ideal model to study aging related mechanisms. In addition, regarding the very special stage of diapause, during which the egg seems to be protected from aging to some extent, researchers are still trying to decipher the complex mechanisms behind this phenomenon. For example, one study investigates if parental aging could affect the egg development and favour diapause (21).
N. furzeri is a relatively new model in ecotoxicological studies with pioneering research conducted to investigate the effects of reference toxic materials like copper on larvae and later stages (22). This work lays the foundation for additional ecotoxicological studies.
Historically, N. furzeri has not been the easiest organism to work with, as it used to present troublesome breeding (male aggressiveness) and unstable hatching. Nevertheless, recent progress in protocols development and standardization opened new possibilities to work with this specie of unique attributes. No current official guidelines regulate the use of killifish eggs and larvae in toxicological studies, as new data is being generated by researchers to validate the use of this model.
Organoids
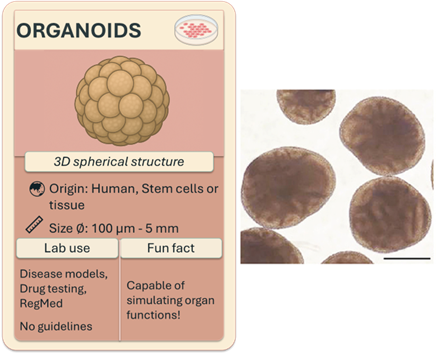
Organoids are self-assembled 3D cell clusters derived from human stem cells or tissues cultured in vitro that mimic human organs. By fine-tuning the biochemical and physical properties of their environment, scientists can direct cell differentiation to generate organ-specific structures: brain, kidney, lung, liver, and many more!
Organoids have gained growing attention in recent years because their origin from human cells allows them to accurately mimic human-specific physiology and disease processes. They can also be customized using patient-derived cells for personalized modelling and drug testing, and their small size (100 µm to 5 mm) makes them ideal for high-throughput screening.
Given their human relevance and scalability, organoids are increasingly explored as a NAM in Toxicity and Efficacy studies for drug discovery:
To assess the organoids potential in drug discovery, some studies compare their sensitivity to reference treatments to the ones of 2D cell cultures or animal models. For example, one study uses intestinal organoids to study the effects of reference antiviral treatments against enterovirus. By comparing the results to established 2D cell culture, organoids have been shown to be more sensitive, highlighting their added value (23).
Organoids offer a valuable model for toxicity testing, as different types can be selected to match the organ of interest. For example, neural organoids are increasingly used to study neurotoxicity by mimicking how harmful substances affect early brain development, potentially leading to conditions like microcephaly or neural tube defects (24). They are also used to investigate neurodevelopmental disorders by examining how environmental exposures, such as neurotoxicants, influence brain development and neuroplasticity (25).
Current challenges involve the translation on in vivo conditions and interactions to organoid models, with limited organ-organ interactions, poor vascularization and simplistic dynamic conditions as typical bottlenecks. Culture scalability is also critical, as reliability, standardization and automation are necessary to fit the pharmaceutical standards. While no regulatory guidelines are currently established, new plans have started to be drafted by official regulatory bodies to support the use of organoids as alternatives to animal models.
Other models
Other emerging NAMs include Caenorhabditis elegans (C. elegans) and Xenopus eggs.
- The nematode elegans is widely used for high-throughput toxicity screening and neurodevelopmental studies due to its simple anatomy, well-annotated genome, and transparent body. Current efforts in research focus on protocol standardization, proposition of guidelines adaptations (26) and scaling up of the model (27).
- Xenopus eggs, especially Xenopus laevis, offer early stage, non-sentient models that can be used for assessing teratogenicity (28) and endocrine disruption, benefiting from their large size, easy handling, external development, and conserved vertebrate pathways. Specific regulations exist for endocrine testing (OECD TG 248, OECD TG 231, OECD TG 241) while specific legal frameworks have not been established yet.

Conclusions
In conclusion, NAMs are steadily gaining traction in regulatory science, offering innovative tools to improve the efficiency, ethics, and scientific relevance of safety assessments. While their regulatory integration remains uneven, NAMs can either replace traditional animal-based tests, when sufficiently validated, or contribute valuable insights as part of a synergistic testing strategy. In this context, Integrated Approaches to Testing and Assessment (IATAs) and the Weight of Evidence (WoE) concept serve as key enablers of NAMs adoption (29, 30).
IATAs provide structured, endpoint-specific frameworks that guide the strategic use of diverse data sources, such as in vitro assays, in silico models, and existing knowledge, tailored to specific regulatory questions. WoE complements this by offering a transparent process to evaluate the quality, consistency, and relevance of those data streams to draw robust, science-based conclusions. Together, IATAs and WoE illustrate how NAMs can be used in combination rather than isolation, forming a cohesive, adaptive, and mechanistically informed approach to decision-making. Continued cross-sector collaboration and international harmonization will be essential to fully unlock the potential of NAMs in shaping the future of regulatory toxicology.
References
- U.S. Food and Drug Administration. (2025, April 10). Roadmap to reducing animal testing in preclinical safety studies (FDA report No. 186092). U.S. Department of Health and Human Services. Retrieved from https://www.fda.gov/media/186092/download
- European Chemicals Agency. (2016). New approach methodologies in regulatory science: Proceedings of a scientific workshop, Helsinki, 19–20 April 2016 (Publication No. ED‑04‑16‑787‑EN‑N; ISSN 1831‑418X, ISBN 978‑92‑9495‑397‑1, DOI 10.2823/543644). Publications Office of the European Union.
- Advisory Committee to the Director, National Institutes of Health. (2023, December 14). Catalyzing the development and use of novel alternative methods (NAMs) to advance biomedical research: ACD Working Group report. NIH. Retrieved from https://www.acd.od.nih.gov/documents/presentations/12142023_NAMs_Working_Group_Report.pdf
- Schmeisser, S., Miccoli, A., von Bergen, M., et al. (2023). New approach methodologies in human regulatory toxicology – Not if, but how and when! Environment International, 178:108082. https://doi.org/10.1016/j.envint.2023.108082
- U.S. Food and Drug Administration. (2025, April 10). FDA announces plan to phase out animal testing requirement for monoclonal antibodies and other drugs [Press release]. U.S. Department of Health and Human Services. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-announces-plan-phase-out-animal-testing-requirement-monoclonal-antibodies-and-other-drugs
- European Chemicals Agency. (2024, March 26). Third workshop to discuss the roadmap to phase out animal testing for chemical safety assessments. https://echa.europa.eu/-/third-workshop-to-discuss-the-roadmap-to-phase-out-animal-testing-for-chemical-safety-assessments
- Mitchell, C. A., et al. (2023). New approach methodologies for the endocrine activity toolbox: Environmental assessment for fish and amphibians. Environmental Toxicology and Chemistry, 42(4), 757–777. https://doi.org/10.1002/etc.5584
- Ball, J., et al. (2025). Determination of the zebrafish embryo developmental toxicity assessment: Evaluating the ZEDTA bioassay for agrochemical prenatal developmental toxicity. Environmental Toxicology and Chemistry. Advance online publication. https://doi.org/10.1016/j.reprotox.2025.108837
- Weiner, A. M. J., et al. (2024). Validation of a zebrafish developmental defects assay as a qualified alternative test for its regulatory use following the ICH S5(R3) guideline. Reproductive Toxicology, 123, 108513. https://doi.org/10.1016/j.reprotox.2023.108513
- Burbank, M., et al. (2023). Advancing the use of New Approach Methodologies for assessing teratogenicity: Building a tiered approach. Reproductive Toxicology, 120, 108454. https://doi.org/10.1016/j.reprotox.2023.108454
- Cornet, C. (2022, November 16). Zebrafish larvae as a new alternative methodology (NAM) for developmental toxicity assessment. ZeClinics. Retrieved from https://www.zeclinics.com/blog/zebrafish-larvae-as-a-new-alternative-methodology-nam-for-the-assessment-of-compounds-teratogenicity/
- https://biobide.com/
- https://www.criver.com/
- https://www.evotec.com/
- https://invivobiosystems.com/
- https://www.creative-biogene.com/
- Oxendine, S. L., et al. (2006). Adapting the medaka embryo assay to a high‑throughput approach for developmental toxicity testing. Neurotoxicology, 27(5), 840–845. https://doi.org/10.1016/j.neuro.2006.02.009
- Liu, J., et al. (2022). Medaka (Oryzias latipes) embryos as a model for metabolism of anabolic steroids. Frontiers in Endocrinology, 13, 854977. https://doi.org/10.1007/s00204-022-03284-4
- Merino, M., et al. (2020). Medaka (Oryzias latipes) embryo as a model for the screening of compounds that counteract the damage induced by ultraviolet and high-energy visible light. International Journal of Molecular Sciences, 21(16), 5769. https://doi.org/10.3390/ijms21165769
- Polačik, M., et al. (2016) Laboratory breeding of the short-lived annual killifish Nothobranchius Furzeri, Nature Protocols, 11(8), pp. 1396–1413. http://dx.doi.org/10.1038/nprot.2016.080
- Api, M. et al. (2018) Effects of parental aging during embryo development and adult life: The case of Nothobranchius furzeri, Zebrafish, 15(2), pp. 112–123. https://doi.org/10.1089/zeb.2017.1494.
- Philippe, C. et al. (2017) Acute and chronic sensitivity to copper of a promising ecotoxicological model species, the annual killifish Nothobranchius furzeri, Ecotoxicology and Environmental Safety, 144, pp. 26–35. https://doi.org/10.1016/j.ecoenv.2017.05.047
- Masmoudi, F., et al. (2023). Evaluation of 3D human intestinal organoids as a platform for EV‑A71 antiviral drug discovery. Cells, 12(8), 1138. https://doi.org/10.3390/cells12081138
- Park, S.-H., et al. (2025). Toxicity assessment using neural organoids: innovative approaches and challenges. Toxicological Research (Korean Toxicology Society Journal), 41(2), 91–103. https://doi.org/10.1007/s43188-025-00279-y
- Alam El Din, D.-M., et al. (2024). Organoid intelligence for developmental neurotoxicity testing. Frontiers in Cellular Neuroscience, 18, Article https://doi.org/10.3389/fncel.2024.1480845
- van der Voet, M., et al. (2021). Towards a reporting guideline for developmental and reproductive toxicology testing in C. elegans and other nematodes. Toxicology Research, 10(6), 1202–1210. https://doi.org/10.1093/toxres/tfab109
- DuPlissis, A., et al. (2025). Machine learning–based analysis of microfluidic device–immobilized C. elegans for automated developmental toxicity testing. Scientific Reports, 15, 15. https://doi.org/10.1038/s41598-024-84842-x
- Menegola, E., et al. (2023). Advantages and disadvantages of the use of Xenopus laevis embryos and zebrafish as alternative methods to assess teratogens. Current Opinion in Toxicology, 34, 100387. https://doi.org/10.1016/j.cotox.2023.100387
- European Medicines Agency. Regulatory acceptance of new approach methodologies (NAMs) to reduce animal use testing. EMA. Retrieved June 23, 2025, from https://www.ema.europa.eu/en/human-regulatory-overview/research-development/ethical-use-animals-medicine-testing/regulatory-acceptance-new-approach-methodologies-nams-reduce-animal-use-testing
- Organisation for Economic Co-operation and Development. (n.d.). Integrated approaches to testing and assessment (IATA). OECD. Retrieved June 23, 2025, from https://www.oecd.org/en/topics/sub-issues/assessment-of-chemicals/integrated-approaches-to-testing-and-assessment.html
