New Approach Methodologies (NAMs) are increasingly used to study biological effects in more human-relevant and ethical ways, yet each model captures only part of the picture. In this month’s article, we explore how combining complementary NAMs, such as 3D cell cultures (e.g. organoids and spheroids) and zebrafish embryos, can help strengthen interpretation and improve confidence in results.
Introduction
In recent years, NAMs have gained increasing attention as innovative tools to generate more human-relevant and ethical insights in biological research and safety assessment. These approaches, ranging from advanced in vitro systems such as organoids to non-mammalian models and in silico methods, have been discussed in previous Bionomous articles as important building blocks for modernizing how biological effects are studied.
In this article, we explore how NAMs are increasingly used alongside one another to address complex biological questions. By looking at current practices and emerging examples, we discuss how combining complementary NAMs can help broaden biological coverage, strengthen interpretation of results, and support more predictive, decision-ready outcomes.
Why and how can NAMs be combined?
NAMs are typically designed to capture biological processes at specific levels of organization. For example, organoids offer detailed insight at the molecular, cellular, and tissue levels, enabling the study of development, disease mechanisms, and responses to chemical or drug exposure in human-relevant systems. In contrast, zebrafish embryos introduce whole-organism complexity, allowing the assessment of systemic effects such as behavior, neurophysiology, and integrated organ responses.
Building on this complementarity, several strategies have emerged to combine NAMs in practice, depending on the research question and context of use:
- In neuroscience research, human brain organoids provide advanced models for neurodevelopment mechanisms, disease modelling, and chemical exposure, but remain resource-intensive and variable, while mouse and zebrafish brain organoids offer more scalable alternatives with reduced complexity. Zebrafish embryos and larvae further add whole-organism context, enabling functional and behavioral readouts that cannot be captured in isolated in vitro systems. Together, and supported by in silico integration, these models strengthen translational relevance and predictive power (1).
- In genetic epilepsy research, zebrafish are used for rapid, phenotype-based drug screening, while iPSC-derived neurons and organoids support target-specific and patient-relevant investigations, together forming integrated discovery pipelines (2).
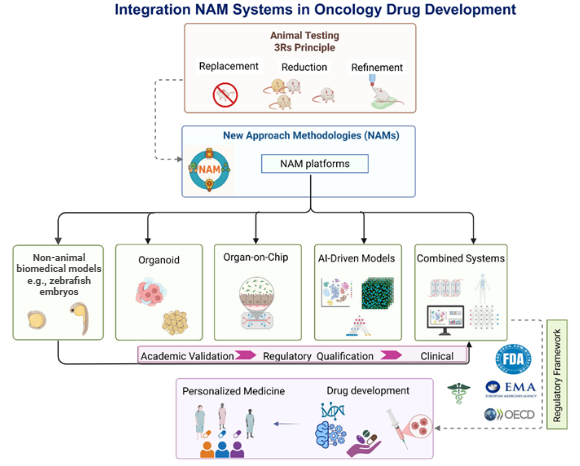
- In oncology, combined NAM approaches help address challenges such as limited clinical translation and tumor heterogeneity. Patient-derived organoids and cancer-on-chip systems are used alongside computational models, often supported by AI, to integrate multi-omic and longitudinal data. Rather than acting as standalone replacements, these tools are increasingly positioned as complementary components of an evidence strategy that supports earlier decision-making and better alignment with clinical questions (3).
NAMs combination: Project examples
Over the past years, several European and international research initiatives have been launched to explicitly explore how combinations of NAMs can be applied to complex human health and environmental questions.
NanoBreak: Human liver organoids and Zebrafish embryos for environmental pollution
NanoBreak is one such concrete example, addressing the growing challenge of environmental nanoplastic pollution (6). Specifically, it targets nanoplastics generated from degraded plastics collected in European coastal areas, better reflecting real-world human and ecological exposure, including both particles and their released chemical additives. The project combines human liver organoids and zebrafish embryos as complementary models. Human liver organoids enable the investigation complex mechanisms occurring in the human liver, like bioaccumulation or metabolic transformation, while zebrafish embryos provide organism-level insight into developmental, systemic, and epigenetic effects. A shared exposure strategy and aligned endpoints allow results from both systems to be directly compared and integrated.
By coupling these models with spatial multi-omics and high-resolution imaging techniques such as Raman spectroscopy, NanoBreak links molecular-level interactions to tissue and whole-organism responses. This integrated approach generates human-relevant data that can support regulatory risk assessment of plastic pollution, contributing evidence applicable to policy frameworks such as the EU Green Deal and the Sustainable Development Goals, and demonstrating how combined NAMs can inform safer material design and environmental protection strategies.
ASPIS Cluster and NAMWISE project: organoid-based and in silico approaches
Other ongoing initiatives illustrate how NAM integration is being pursued across Europe, particularly by combining organoid-based and in silico approaches.
For example, the ASPIS Cluster (“Animal-free Safety assessment of chemicals: Project cluster for Implementation of novel Strategies”) brings together several projects dedicated to advancing chemical safety assessment using complementary in vitro and computational methods, including organ-on-chips systems, integrated omics and AI-driven modelling to map toxicity pathways and support next-generation risk assessment strategies (7).
Similarly, the NAMWISE project aims at developing practical guidance on how to validate, integrate, and use combinations of in vitro (including organoids and organ-on-chip) and in silico methods within regulatory evaluation frameworks for chemicals and pharmaceuticals. Its objectives include addressing regulatory implementation challenges, defining standardisation needs, and delivering case studies through a collaborative framework that brings together partners from 8 European countries, spanning industry, NAM developers, regulators, academia, public research institutes, and consulting organizations across both the chemical and pharmaceutical sectors (8).
PrecisionTox: human cell lines, biomedical model organisms and AI as a new method to study toxicology
Another example of combined NAMs is PrecisionTox, an EU-funded project that is part of the ASPIS Cluster. The project explores a “precision toxicology” approach by bringing together human cell-based systems with a range of non-mammalian models, including zebrafish and frog embryos as well as invertebrate organisms like fruit flies, water fleas and round worms. By leveraging evolutionary conservation across species and integrating AI approaches, PrecisionTox aims to identify how chemical exposures trigger toxicity pathways and to translate these mechanistic insights into more predictive assessments of human and environmental health risks. The project illustrates how integrating complementary NAMs can generate regulatory-relevant evidence while supporting the EU’s broader goals for reducing animal testing.
As combined NAM strategies mature, the focus is shifting from proof-of-concept to practical use. This naturally brings regulatory considerations to the forefront, particularly how integrated NAM evidence should be validated, contextualized, and compared.
What does this mean for regulatory acceptance?
If combining NAMs can deliver richer and more predictive insights, an obvious question follows: how are these approaches actually evaluated for regulatory use? How should integrated NAM evidence be assessed and compared to animal studies, especially when animal models themselves have known limitations?
Validation beyond animal comparison
Regulatory thinking is increasingly moving away from direct one to one comparison with animal models. Several expert groups now emphasize that animal data should not automatically be treated as a gold standard, since many animal endpoints do not reliably predict human outcomes (9). Instead, validation is becoming fit for purpose, meaning that methods are assessed based on how well they answer a defined regulatory question. For combined NAM strategies, this involves evaluating biological relevance, mechanistic plausibility, and consistency across models, rather than simple concordance with animal outcomes.
Context of use is key
Closely linked to validation is the definition of context of use. A NAM combination designed for early hazard identification, screening, or mechanistic insight is not expected to serve the same role as a method intended for quantitative risk assessment. Clearly defining the question being addressed helps regulators interpret integrated NAM evidence appropriately and increases confidence in its use.
Reproducibility and comparability
Reproducibility remains a core requirement for regulatory acceptance. For combined NAMs, this means demonstrating robustness across laboratories, aligned exposure designs, and standardized data recovery. Automation and digital analysis tools, such as the EggSorter, can support this process by reducing operator variability and improving transparency and traceability of complex datasets, which are increasingly important for regulatory confidence.
Conclusions & Outlooks
Overall, combining NAMs holds great promises to provide richer and more predictive evidence than single models. The focus is now on fit-for-purpose validation, clear context of use, and reproducibility, rather than direct comparison to animal tests.
This year, several regulatory workshops and conferences will provide opportunities to advance these discussions, including sessions on integrated NAM approaches and standardization. Continued collaboration between researchers, regulators, and industry will be key to ensuring that NAM combinations are credible, decision-ready, and increasingly adopted in regulatory frameworks.
References
- Imberechts, D., Ny, A., & Copmans, D. (2025). Established and emerging new approach methodologies in neuroscience. Frontiers in neuroscience, 19, 1696937. https://doi.org/10.3389/fnins.2025.1696937
- Shcheglovitov, A., Peterson, R.T. Screening Platforms for Genetic Epilepsies—Zebrafish, iPSC-Derived Neurons, and Organoids. Neurotherapeutics 18, 1478–1489 (2021). https://doi.org/10.1007/s13311-021-01115-5
- Mirlohi, M. S., Yousefi, T., Aref, A. R., & Seyfoori, A. (2025). Integrating New Approach Methodologies (NAMs) into Preclinical Regulatory Evaluation of Oncology Drugs. Biomimetics (Basel, Switzerland), 10(12), 796. https://doi.org/10.3390/biomimetics10120796
- Jaeschke, H., & Ramachandran, A. (2025). Are New Approach Methodologies (NAMs) the Holy Grail of toxicology? Toxicological Sciences, 208(1), 1–8. https://doi.org/10.1093/toxsci/kfaf113
- Bearth, A., Roth, N., Jansen, T., Holden, L., Čavoški, A., Di Consiglio, E., Hauzenberger, I., Lee, R., Mombelli, E., Tcheremenskaia, O., Wendt-Rasch, L., & Wilks, M. F. (2025). New approach methodologies in human health risk assessment across European regulatory frameworks: Status quo, barriers and drivers for regulatory acceptance and use. Environment International, 196, 109279. https://doi.org/10.1016/j.envint.2025.109279
- National Institute of Biology. NanoBreak – Breaking the plastic chain: Unraveling the pathways and cellular responses to environmentally aged nanoplastics. https://projects.nib.si/nanobreak/
- ASPIS Cluster. [Project cluster for implementation of novel strategies to advance chemical safety assessment]. Retrieved from https://aspis-cluster.eu/the-projects/
- NAMWISE. NAMs Within Integrated Safety & Efficacy evaluation of chemicals and pharmaceuticals [Horizon Europe project]. Retrieved from https://namwise.eu/namwisethe-project/
- Ouédraogo, G., Alépée, N., Tan, B., & Roper, C. S. (2025). A call to action: Advancing new approach methodologies (NAMs) in regulatory toxicology through a unified framework for validation and acceptance. Regulatory Toxicology and Pharmacology, 162, 105904. https://doi.org/10.1016/j.yrtph.2025.105904